What is Essure?
Essure is a non-surgical alternative to laparoscopic sterilization (tubal ligation) for all those women who seek permanent birth control. This device does not require hormones nor surgical incisions to work, and it consists of a couple of small metal coils that are inserted into the fallopian tubes. The coils are made of Nickel titanium, stainless steel, and polyethylene fibers and require just a quick day-hospital intervention to be implanted. After the device is properly positioned inside the tubes, scar tissue starts building up because of a natural inflammatory reaction. After about three months, the scar tissue completely blocks the tubes, effectively sealing them and preventing sperm cells to reach the ovaries. This procedure is called hysteroscopic sterilization and has no effects on the menstrual cycle.
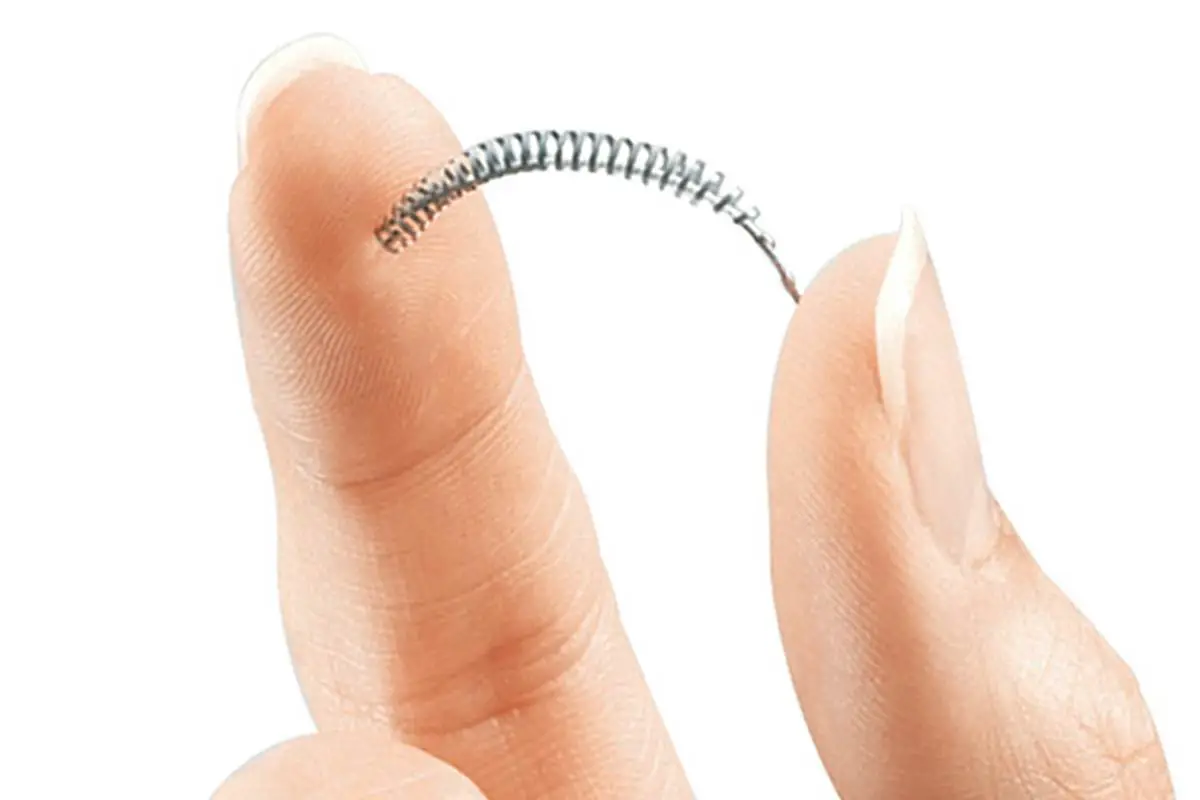
Is this device safe?
Many women who were implanted with this device started complaining about all kind of side effects, ranging from excruciating abdominal and back pain to bloating, weight gain, constant migraines, depression, chronic fatigue, and sexual dysfunction. Since Essure received its first market approval in 2002, almost 10,000 adverse reaction reports have been filed by patients and doctors as well. The inflammatory response required to form the scar tissue around the coils could be much more detrimental to one’s health than manufacturer’s claimed, leading to many unexpected consequences. Many women also risked their lives after their bodies rejected the device because of a possible nickel allergy or hypersensitivity that researchers never accounted for during clinical trials.
The most severe complications and death reports
Some of the women who were implanted with this device suffered much more serious harm, and in some cases, they even died because of the consequences.
Amanda Dykeman, a 28 years woman from Illinois, was diagnosed with chronic inflammation of the cervix. After three years since the implantation, the coils perforated her tubes, leading to the formation of a series of life-threatening blood clots. Even after her uterus was completely removed (hysterectomy), Mrs. Dykeman’s abdominal organs formed many adhesions, causing her severe digestive issues.
In 2012, Becky Beesley was reoperated shortly after the device was implanted. The coils did, in fact, broke, and the pieces started migrating through her body. To avoid more potentially lethal consequences she had to undergo a total hysterectomy.
Amanda Holt, a woman from Phoenix, was operated after she started complaining about an excruciating and “horrible pain” that prevented her from performing any activity. After device breakage, one of the coil’s splinters lodged in her abdominal lining, requiring a very delicate operation to remove it.
The U.S. Food and Drugs Administration (FDA) received hundreds of similar reports of device migration, breakage and organ perforation. Some of the worst adverse reaction reports, however, indicated that the patient died either because of the physical complication, nickel allergies, or because they suicided after falling into depression.
Essure side effects
- Back, neck and abdominal pain
- Heavier or irregular menstruation
- Gastrointestinal issues
- Nausea and vomiting
- Chronic fatigue
- Constant pain
- Device migration
- Device Breakage
- Organ perforation
- Bloating
- Sexual dysfunction
- Bleeding
- Nickel allergy and anaphylaxis
- Leg cramps
- Unusual vaginal discharges
- Weight gain
- Depression
- Blood clots
- Mood swings
- Suicidal thoughts
- Death
Can Essure be removed after implantation?
Although removing this device is actually possible, it still is all but an easy procedure since there’s a chance that some small metal fragment can remain lodged in the nearby tissues. Women who require Essure removal have two choices available: a delicate procedure to perform. The first one is a technique the surgeon to cut a small-sized hole in the fallopian tubes (salpingotomy) to remove the coil. Although minimally invasive, this technique is extremely complicated uncommon and require an extremely specialized surgeon that is usually quite rare to find. The other alternative is complete removal of the entire uterus, cervix and fallopian tubes (hysterectomy). This technique will remove most of the female reproductive apparatus, leaving the woman unable to conceive for the rest of her life. It may also cause additional health issues such as incontinence and organ prolapse.
Is there a risk of becoming pregnant after being implanted with Essure?
According to manufacturer’s data, this device is 99.83 percent effective at preventing pregnancy. However, a study published in the journal Contraception that examined the effectiveness of Essure in real-world conditions provided convincing evidence that this percentage is highly inflated. Non-perfect placement of the device, as well as an incomplete blockage of the fallopian tubes, are some of the not-so-infrequent conditions that may increase the risk of an unexpected pregnancy. Also, Conceptus’ researchers did not examine women younger than 21 or older than 45, neither they followed up them for more than five years. Real-world data indicated that up to 96 women out of 1,000 (a little less than 10%) would get pregnant within ten years, which is four times more than the risk of pregnancy associated with laparoscopic sterilization (about 2.5%).
Controversial clinical data
FDA gave approval to Essure with the fast track process in 2002, although they specified that Conceptus had to follow patients for five more years to monitor them about the long-term safety of the device. The trials examined back then were, in fact very small, and followed women for just two years. The manufacturer, however, did not disclose data from their longer clinical trials in 2007 as they were expected to. FDA had to wait until April 2015 to see them, more than eight years later.
In the meanwhile, a larger study published in 2015 in the British Medical Journal showed that women who underwent hysteroscopic sterilization were at a 10-fold higher risk of ending back in the operatory room than those sterilized with different methods. What’s worse, is that the surgical intervention required during reoperation is usually very complicated and risky, much more than the same laparoscopic intervention that women tried to avoid when they chose this “safer” birth control strategy.
Erin Brockovich and her fight against women discrimination
In the last decade, thousands of women shared their stories about Essure complications and side effects on the internet. A group of them founded a Facebook group that later grow to incredibly vast proportions, giving birth to the so-called “E-sisters” movement. Their aim was to inform as many women as possible about the risks associated with this device by publicly talking about the experiences. The famous consumer advocate Erin Brockovich started her own campaign in 2014 to defend women’s rights against the discrimination that she claims many women suffered. According to her, Bayer dismissed all patients’ complaints as the irrational grievances of “hormonal little women.” Representatives from the pharmaceutical company did, in fact, explained that “science must guide them” against all the “emotions involved.”
FDA issues the “Black Box” warning
After receiving almost 10,000 total adverse reaction reports, in September 2015, the FDA issued an investigation panel to determine whether an Essure recall was required and to reevaluate the device’s safety profile. On February 2016, the surveillance assessment was completed, and the regulator found that the device was significantly riskier than it was initially determined. Real-world data did, in fact, show that the new birth control method was associated with so many side effects, that they issued the “Black Box” label to warn the patients. The FDA also requested the device manufacturer’s to follow 2,000 women for an additional period of 3 years to further investigate on its safety and effectiveness. Patients and doctors must also sign an informed consent before Essure could be implanted.
Has Bayer falsified data during clinical trials?
In two different instances, rumors have circulated about Bayer and Conceptus altering the data about Essure’s safety. NBC 4 News New Yok reported that the pharmaceutical giant altered the records to achieve FDA’s approval. Several women such as Kimberly Hock and Gabriella Avina did, in fact, claim that clinical staff did not properly record the feedback they provided whenever they suffered from complications during the trials. According to these women, Conceptus researchers discontinued their follow-up recording as soon as they started complaining about issues.
After completing February 2016 review instead, the FDA found that at least 300 adverse event reports were filed incorrectly. In most of them, the reporter was mislabeled as “other” instead as “physician”. This mistake may have had serious repercussions on the final safety evaluation outcome. If a report was filed by a healthcare provider such as a doctor, pharmacist or nurse, it holds much more weight than if a patient filed it. Although after a short controversy the FDA assumed all responsibilities for the error blaming a “software glitch,” if Bayer actually provided the regulator with skewed data on purpose it could be held liable of concealment.
